Certification
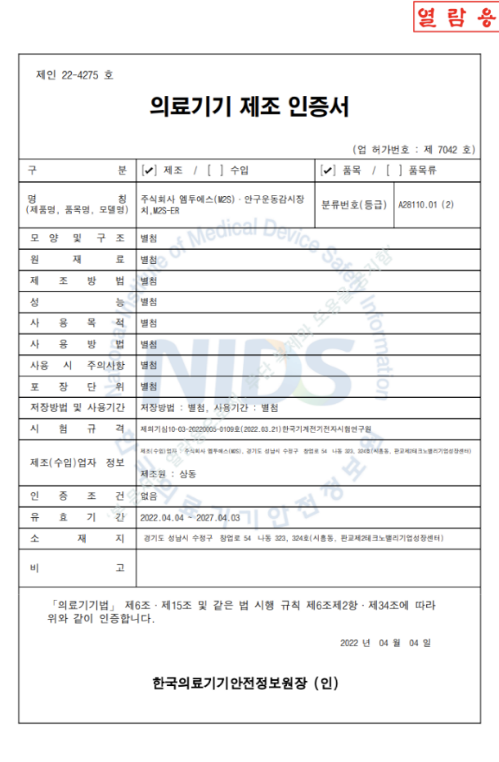
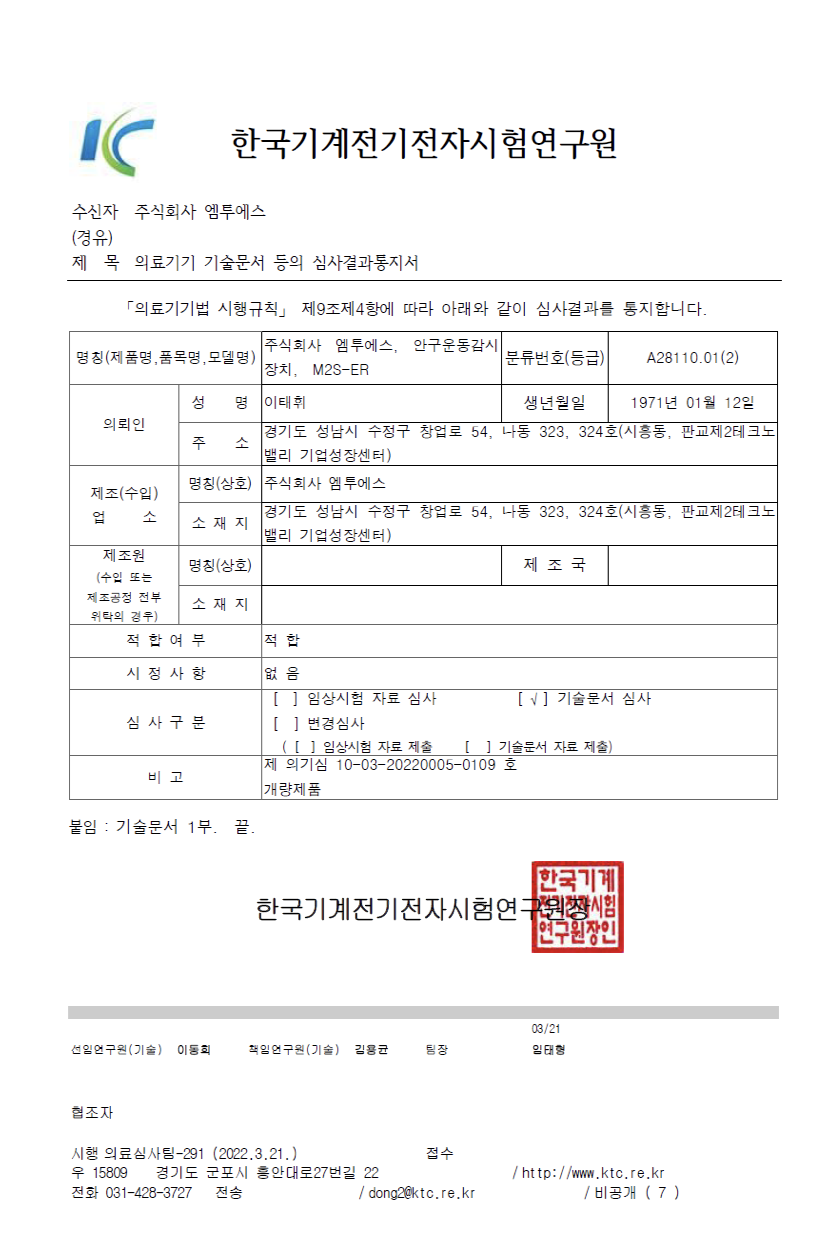
Product name: Eye movement monitoring device, Monitor, eye movement
Model Name: M2S-ER
Classification No.: A28110.01
Classification Level: Class II
Item Definition: Measurement of eye movement. recording device
Certification date: April 4, 2022
Approval
Certification standard: KSP ISO 13485:2016/ISO 13485:2016
Scope of certification: design, development and structure of eye movement monitoring device software
Certification date: June 20, 2022
FDA registration
Registration number: 10061794.
Device Listing number: D362543,
D414762, D414763, D414764,
D414766, D414768
SCI thesis
Nam HY, et al. Neurology, 2021, Pearls & Oysters: Labyrinthine infarction mimicking vestibular neuritis (IF: 11.8)
Kim KT, et al. J Neurol, 2021, Primary ventriculitis presenting with isolated vestibular syndrome (IF: 6.7)
Park YB, et al. J Neuroophthalmol, 2021, Bilateral internuclear ophthalmoplegia as a manifestation of varicella zoster encephalitis (IF: 4.4)
Kim KT, et al. J Neurol, 2021, Punctuate hippocampal lesions presenting with acute vestibular syndrome (IF: 6.7)
Kim JG, et al. J Neurol, 2022, Head‑impulse tests aid in differentiation of multiple system atrophy from Parkinson’s disease (IF: 6.7)
Kim KT, et al. Neurology, 2022, Clinical Reasoning: A 48-Year-Old Woman Presenting With Vertigo, Ptosis and Red Eyes (IF: 11.8)
Kim SH, et al. J Neuroophthalmol, 2022, Triquarter Visual Field Defect Due to Internal Carotid Artery Giant Aneurysm (IF: 4.4)
Hong JP, et al. J Neurol, 2022, Pseudo‑pupil sparing oculomotor nerve palsy in cavernous‑carotid fistula (IF: 6.7)
Kim JG, et al. Clin Aut Res, 2022, Utricular dysfunction in patients with orthostatic hypotension (IF: 5.6)
Kim HJ, et al. Ophthalmology, 2022, Isolated Inferior Rectus Palsy due to Oculomotor Dorsal Subnucleus Infarction (IF: 14.3)
Kim SH, et al. J Neurol, 2022, Alternating adduction hypertropia as a rare presentation of midbrain hemorrhage (IF: 6.7)
Lee SU, et al. J Neurol, 2022, Discordant horizontal–torsional nystagmus: a sign of posterior semicircular canal dysfunction (IF: 6.7)
Lee SU, et al. Front Neurol, 2022, Commentary: Is There an “Acquired Idiopathic Head-Shaking Nystagmus?”: A Discussion of
Mechanisms and Clinical Implications Based on a Case Report (IF: 4.1)
Lee SU, et al. J Neurol, 2022, Acute comitant strabismus in anti‑GQ1b antibody syndrome (IF 6.7)
Kong J, et al. J Clin Neurol, 2022, Isolated Bilateral Superior Cerebellar Peduncular Lesion Presenting Square-Wave Jerks and Ataxia (IF: 2.6)